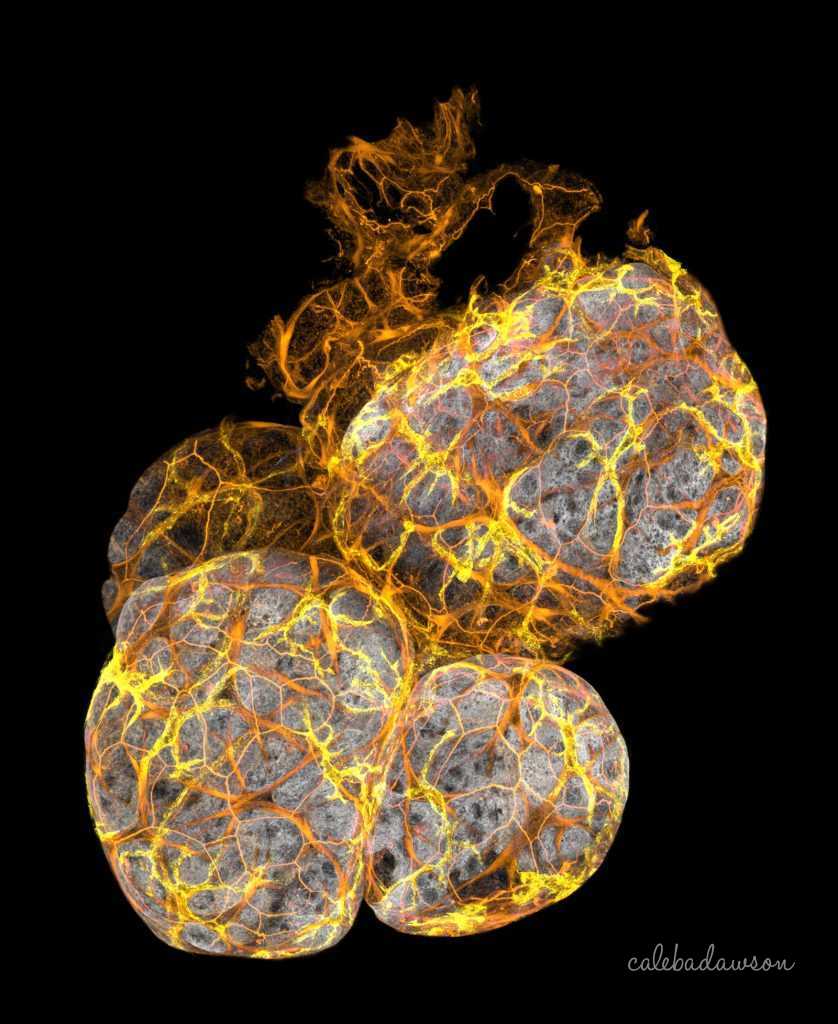
Discovery of new immune cells in breast ducts
The mammary gland is beautiful – it branches like a tree and forms incredible blooming structures that wax and wane with the reproductive cycle. Epithelial cells specialize in forming thin layers, like in the lung and intestine. In the breast, they form thin ducts that make and transport milk in lactation.
Breast cell types have diverse roles and shapes that fit together and work cooperatively. They respond to hormones so that they only grow and make milk when necessary. Unfortunately, the ability of mammary epithelial cells to grow and change makes them a frequent target of cancer.
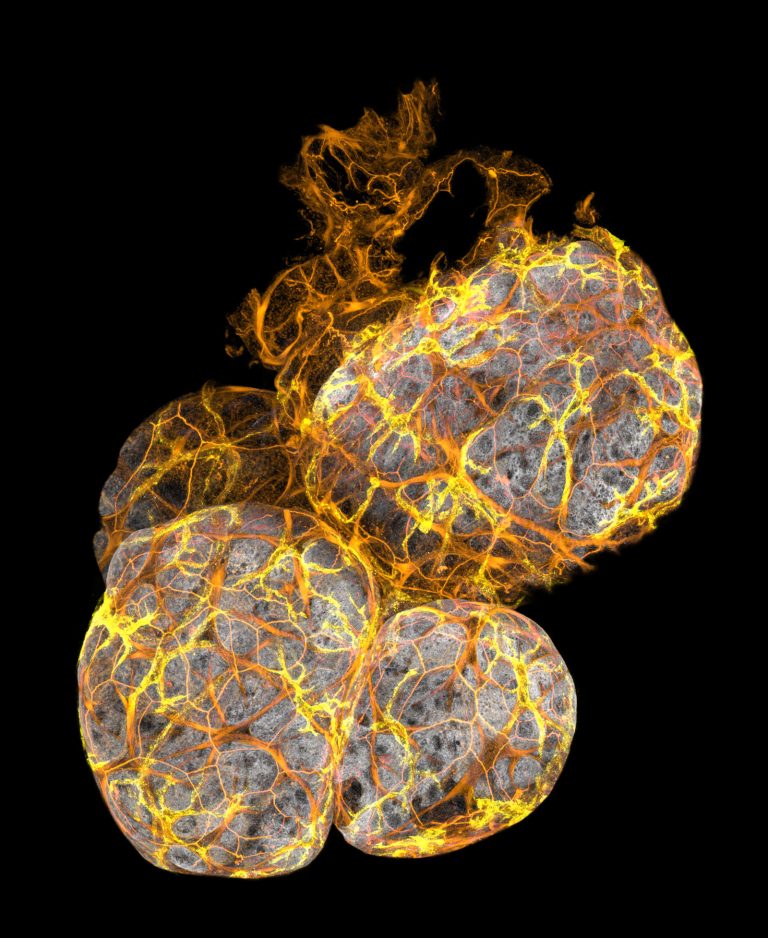
3D Imaging: This is an image of mammary ducts was taken by 3D confocal microscopy. The breast cells had a gene that increases the risk of breast cancer, which caused all of the small branches to form. This type of imaging has led to the discovery of a new type of immune cell in the mammary gland, published in the journal Nature Cell Biology. The research was led by myself, Prof. Jane Visvader, Dr Anne Rios and Prof. Geoff Lindeman at the Walter and Eliza Hall Institute; Find more information here.
Below, I’ll explain how we made this discovery, while taking you for a tour of the natural artworks hidden inside the breast.
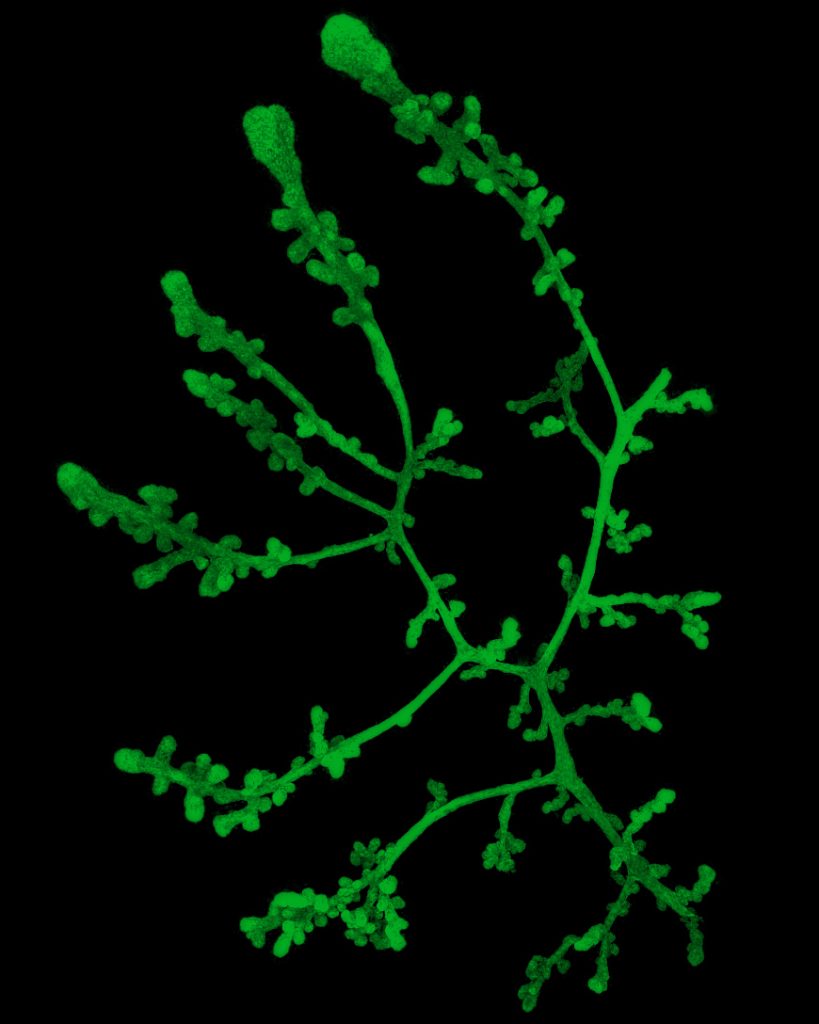
3D exploration of mammary gland structure
Mammary ducts don’t exist in a void: they are embedded in fat that holds blood vessels, connective tissue and immune cells. During my PhD, I used 3D imaging to explore how the diverse cells of the breast interact with the ducts, asking:
Are there important cell relationships that we haven’t discovered yet?
If you find this interesting and helpful, please support me on Ko-fi.
3D imaging of a mammary duct stained for cell skeletons (F-actin, red), myoepithelial cells (Keratin 5/K5, purple) and cell-cell connections (E-Cadherin, yellow).
Finding new cell interactions
We looked at nerves and immune cells because not much is known about where they’re located in breast tissue. Looking at this 3D animation was the first time that I spotted some interesting-shaped immune cells near the ducts…. what are they?
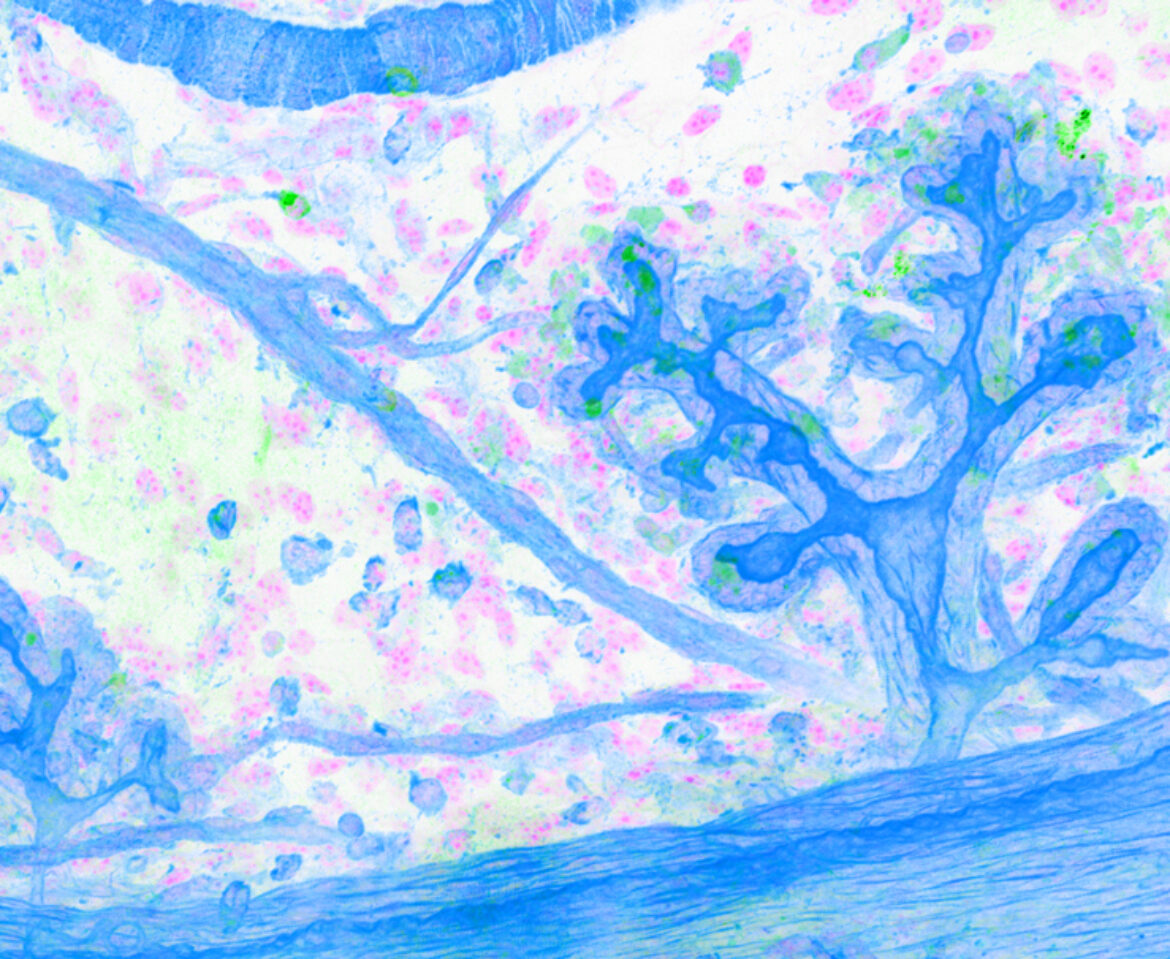
3D imaging of mammary ducts stained for cell skeletons (F-actin, grey), showing nerves (B3tub, thin yellow lines) and then immune cells (CD45, round yellow cells).
Piecing together the mammary puzzle
To see how these immune cells fit into the mammary puzzle, let’s look at the duct structure. The duct wall has two layers that are tightly attached. The cells of the inner layer are round-ish and form a sheet. The outer cells are elongated and lie lengthwise along the duct. Just beyond the duct is a layer of collagen that separates mammary cells from all the others.
3D imaging of a mammary duct showing the elongated myoepithelial cells (K5, green) and inner luminal layer (E-Cadherin, cyan).
An immune cell sandwich
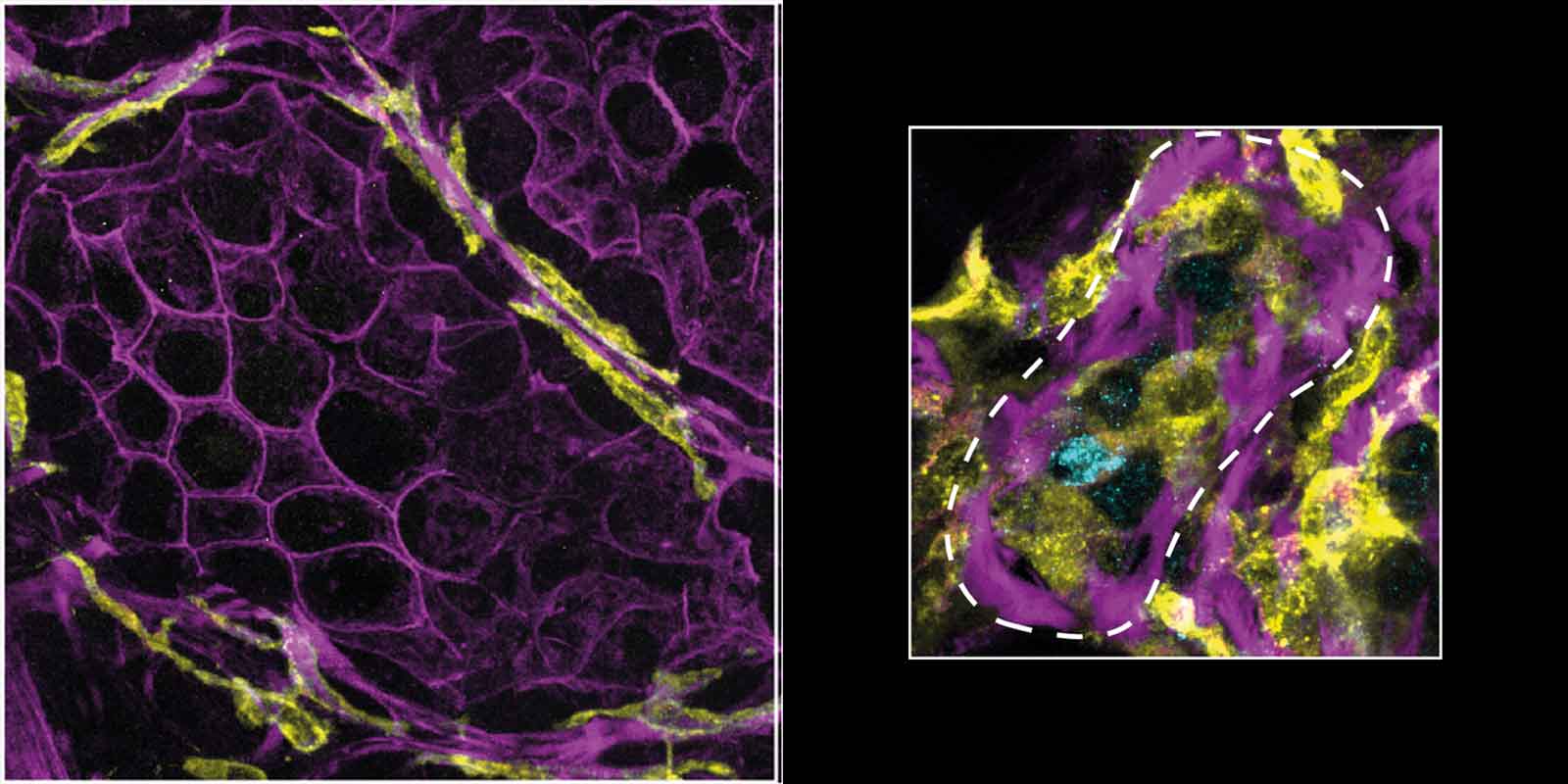
I took some closer images of the immune cells and the duct layers… they are sandwiched INSIDE in the duct wall!
Finding immune cells within the duct was totally unexpected.
At this point, I had to go and learn a lot about immune cells because I had no idea what to do next. Thankfully there were heaps of great immunologists around to help me out.
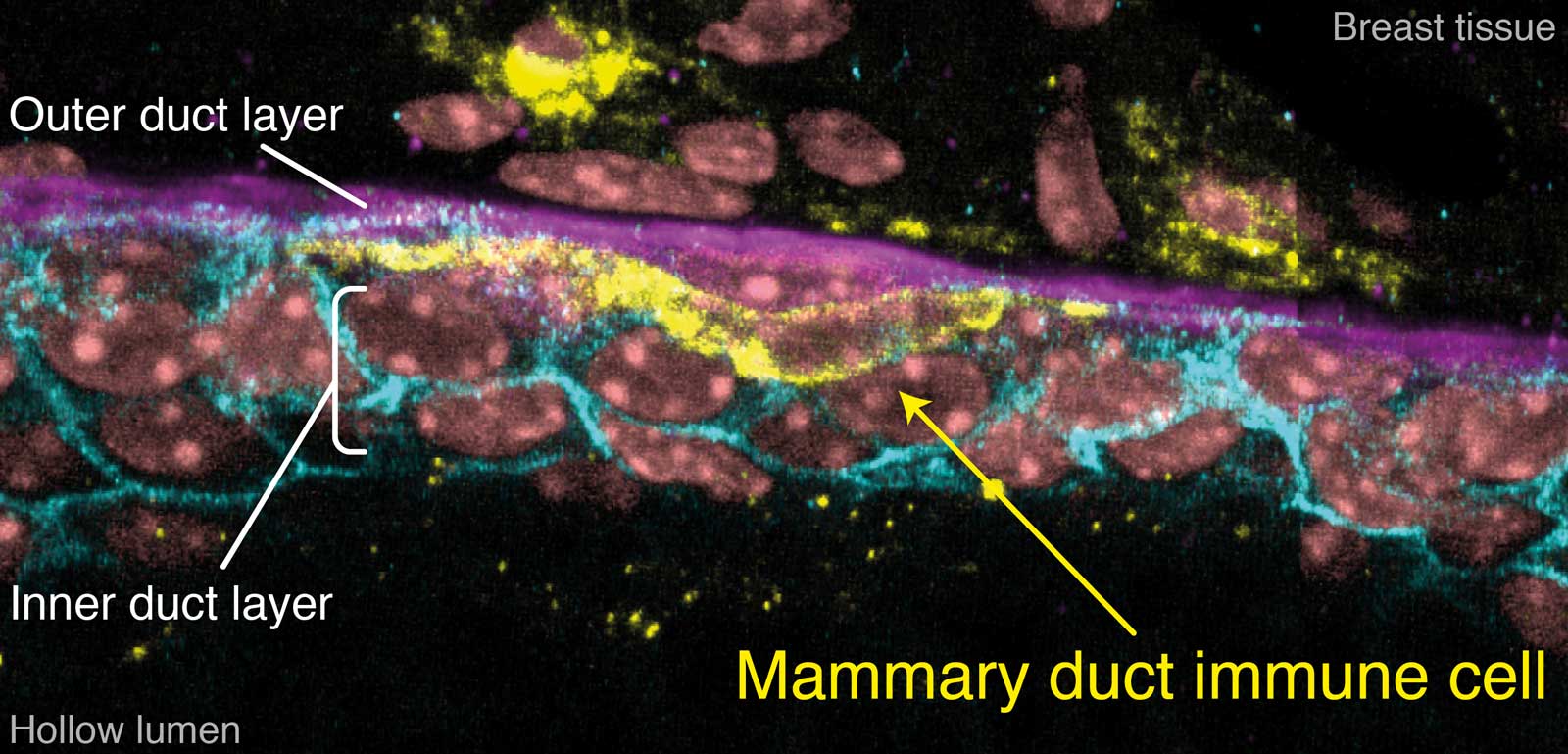
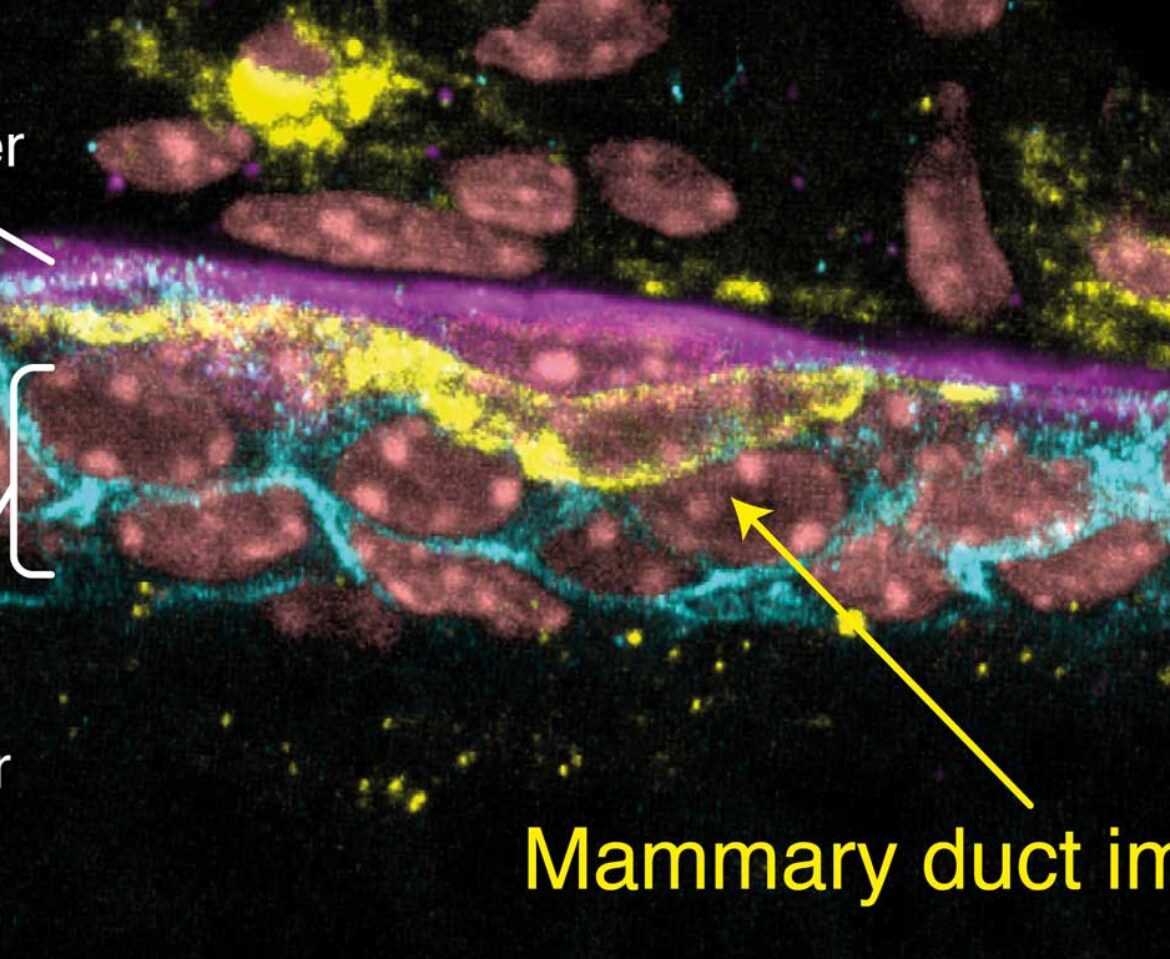
A slice through a mammary duct from a 3D image, stained for inner layer cell-cell connections (E-Cadherin, cyan), outer myoepithelial cells (K5, purple), immune cells (MHCII, yellow) and DNA (DAPI, pink).
Discovering more about duct immune cells
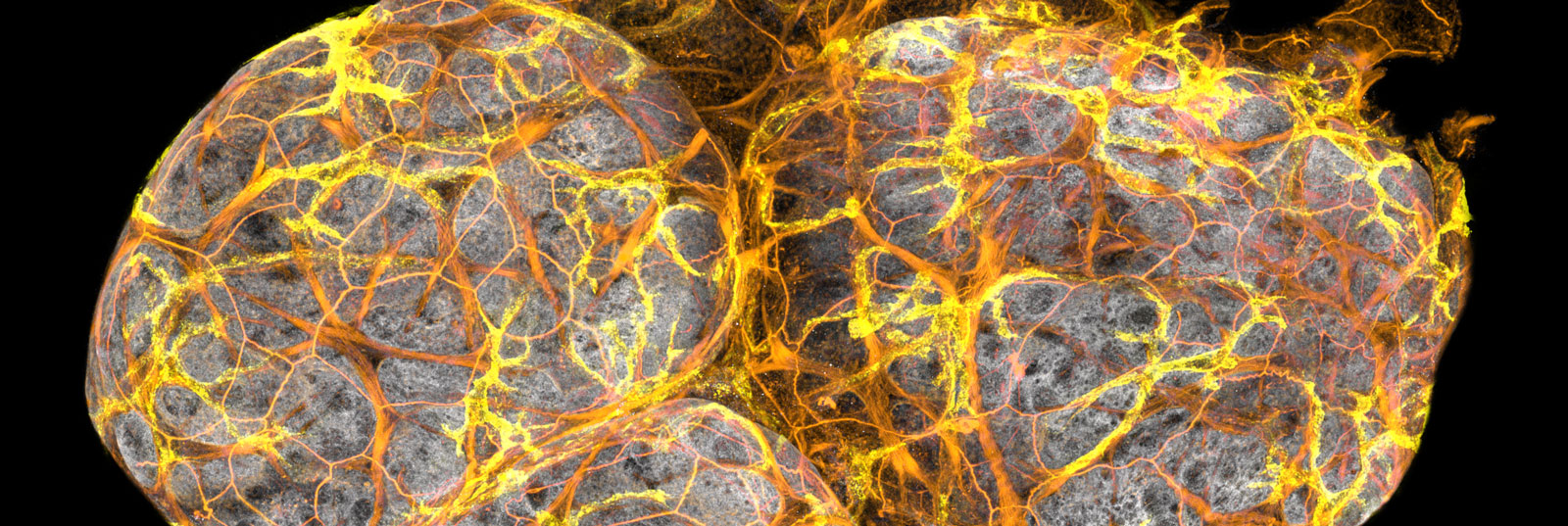
These duct immune cells express genes that define a type of immune cell called macrophages (Csf1r and Cx3cr1), which come from the Greek words for ‘big eater’. Staining for Cx3cr1 before imaging the ducts revealed just how many there were inside the ducts – they cover the entire tree!
These cells are unique to the mammary ducts so we called them ductal macrophages, or ‘DMs’.
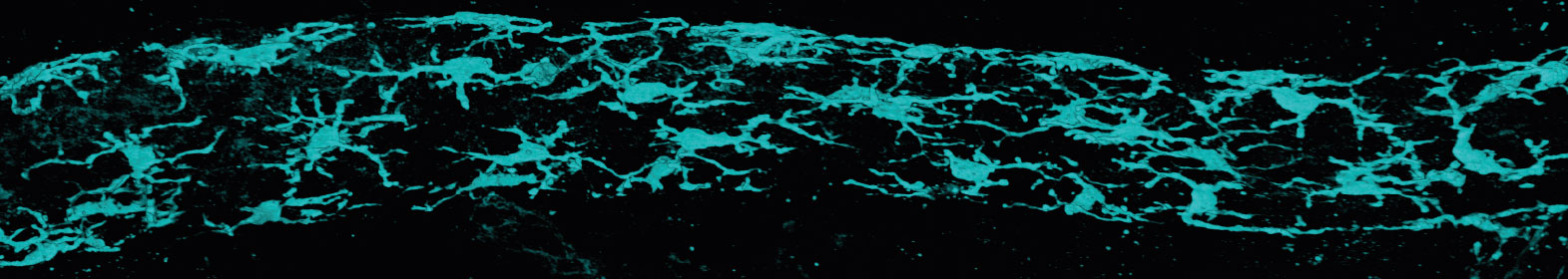
A 3D image of ductal macrophages (Cx3cr1-GFP) forming a network within a mammary duct.
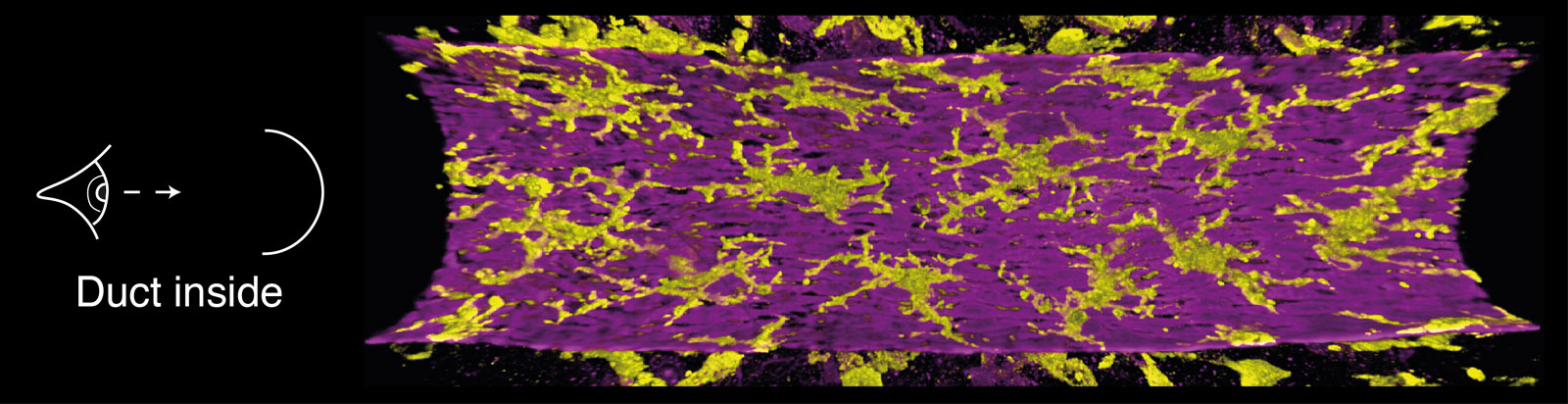
A 3D image of ductal macrophages (MHCII, yellow) underneath the outer myoepithelial layer (K5, purple) of a mammary duct.
Capturing DM behaviour
Now that we had found what the duct immune cells were, we set out to film them. We designed a new way of imaging mammary glands in the lab to get the clearest footage of breast cell behaviour yet.
DMs have this incredible behaviour, where they stay in the same position but move their arms back and forth surveying the duct.
Tracking how much duct they contact showed that DMs touch all cells in the breast every few hours.
Imaging of ductal macrophages (MHCII, yellow) and other immune cells (MHCII, blue) on top of inner layer cells (Elf5-GFP, purple) and collagen (pink).
What about lactation?
The gland is completely transformed in lactation as alveoli form on the ducts to resemble grapes on a vine. We looked at the number of DMs on the alveoli and found 40 times more than in the resting gland.
DMs divide as the gland grows in pregnancy, making more of themselves so that they cover the alveoli in lactation.
3D imaging and animation of lactating breast tissue stained for myoepithelial cells (K5, purple), immune cells (MHCII, yellow) and cell skeletons (F-actin, pink).
Why are there so many DMs in lactation?
DMs might have an unknown role during lactation, but we focussed on the events after lactation during weaning. Milk-producing cells are created during pregnancy and when they are no longer needed, they die. This process is like leaves falling off a tree before winter and is called involution. Do DMs live up to their namesake during involution and feast?
We saw that DMs fill the alveoli just as the milk-producing cells are dying.
This was a big hint that DMs might do exactly that.
Slices from 3D images of mammary glands in lactation (left) and involution (right) stained for cell skeletons (F-actin, purple), immune cells (MHCII, yellow) and dying cells (CC3, cyan). The alveoli collapse due to death of the milk-producing cells (round hollow shapes) and are filled by DMs.
Catching DMs eating
How can we tell if DMs are actually eating the cells? Luckily, we have mammary glands with a fluorescent protein in the milk-producing cells – we only need to look for fluorescent DMs.
We were able to image and film DMs wrapping themselves around milk-producing cells.
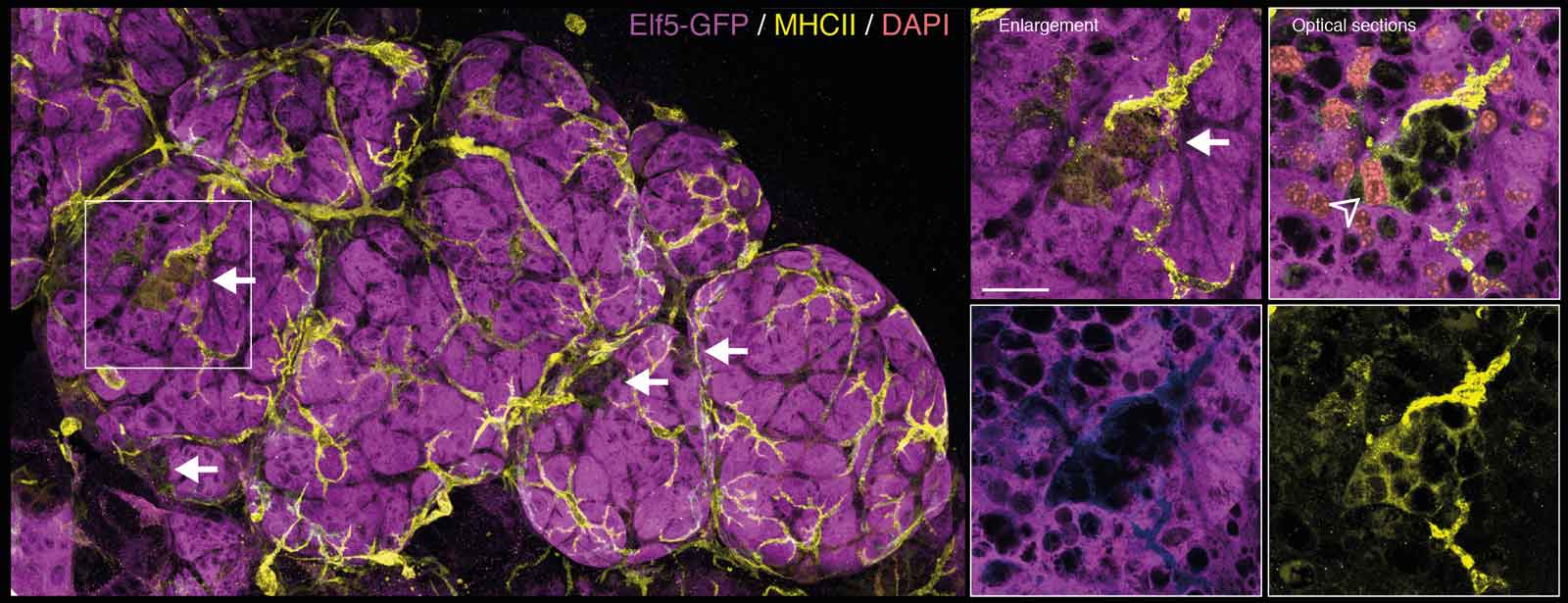
A 3D image during involution, just before alveolar collapse, showing milk-producing cells (Elf5-GFP, purple) and DMs (MHCII, yellow). The arrows point to DMs that have wrapped themselves around the large purple cells that have two nuclei (DNA/DAPI, pink), a unique feature of milk-producing cells.
What happens when DMs are missing?
To know whether DMs are important for involution, or if other cells can easily do the job, we had to get rid of them. We found ways of removing DMs while leaving other immune cells in place.
Without DMs, milk-producing cells weren’t cleared away and alveolar collapse was delayed.
DMs are the only immune cells able to remove milk-producing cells after lactation. They are strategically positioned, ready and waiting to respond to this dramatic remodelling.
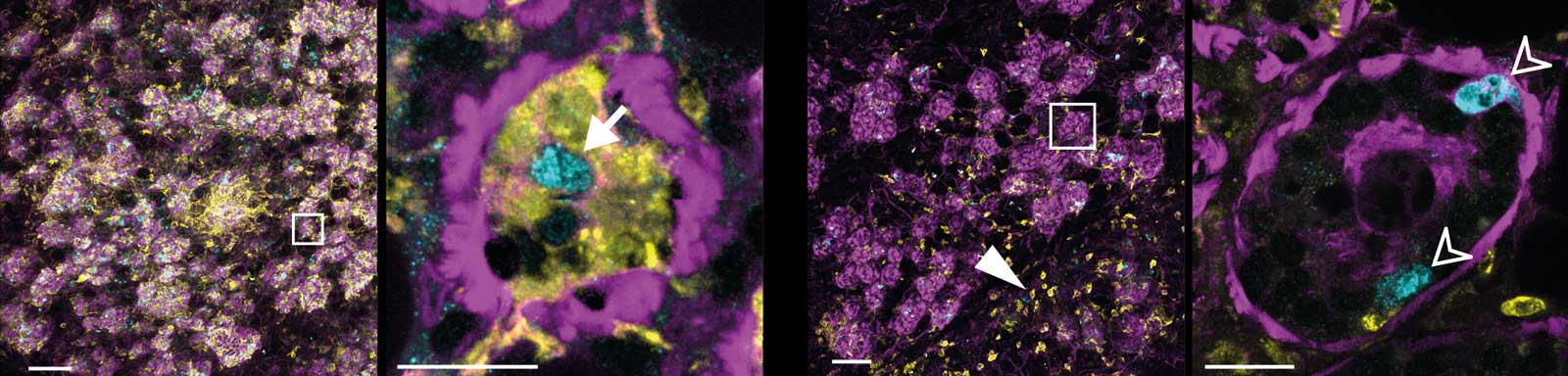
3D images of involuting breast tissue with (left two images) and without (right two images) DMs (yellow). Purple cell skeletons (F-actin) show the shape of the alveoli that are more collapsed when DMs are present. Dead cells (CC3, cyan) are eaten by DMs (arrow) but are left uneaten (hollow arrowheads) when DMs are removed. Scale bars, 100 µm (overviews) and 20 µm (enlarged white boxes).
Finding the mysterious origin of breast cancer macrophages
The reason why we research the normal mammary gland is to better understand where breast cancer comes from and how we can improve treatments.
Macrophages aid breast cancer by providing growth and migration signals, and by dampening the body’s immune attacks against the cancer.
The mystery is that breast tumour-associated macrophages (TAMs) are really different from normal breast tissue macrophages. We compared the molecular features of breast TAMs to DMs and other breast macrophages.
Breast cancer macrophages and DMs are alike. We think normal and cancerous cells ‘teach’ macrophages in the same way.
These macrophages even look the same, lying inside the tumour with many small arms extended to touch the cancer cells.
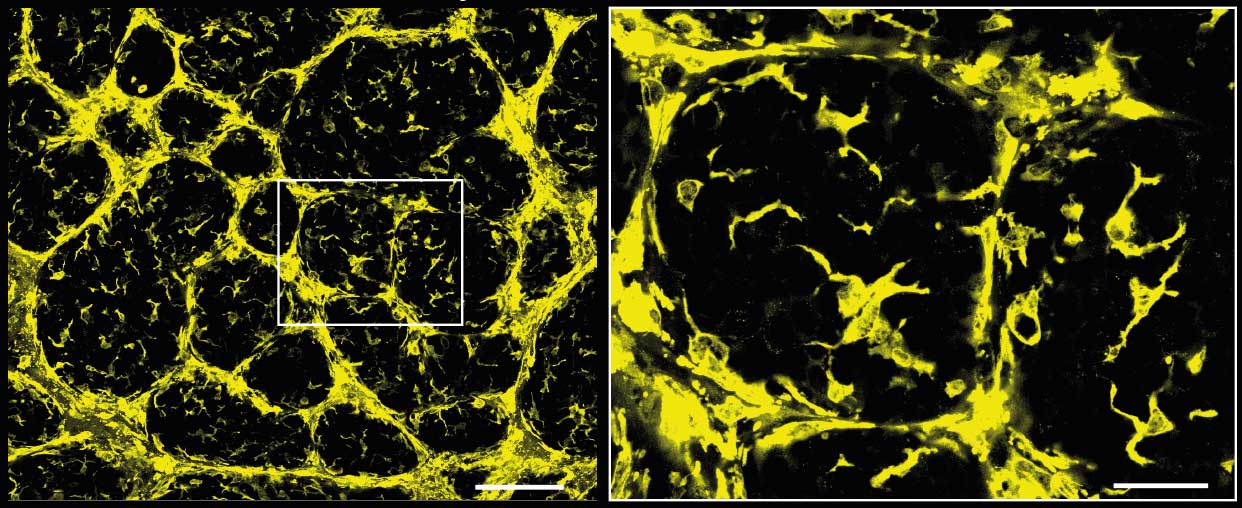
A slice from a 3D image of a breast tumour showing macrophages (Cx3cr1-GFP). The dark circles are where the tumour cells have formed large clumps – inside these, there are star-shaped macrophages that resemble DMs.
Where to now?
There are so many questions still to be answered. This is the great thing about science: that we can always go deeper and learn more about ourselves and our world.
Do DMs help or hinder early breast cancer?
Do DMs help the mammary gland to grow in puberty and pregnancy?
What signals do the ducts and DMs send to each other?
What happens when we block their communication?
While science is never-ending, I love to rest sometimes and reflect on how much we already know about our incredible world. Why is it so beautiful and why do only humans have the ability to understand and appreciate it?
Big questions
I believe in a God who loves people and wants us to know him. Does he prepare natural wonders for us so that we might think of how it came to be, and turn our eyes to beyond what we can see?
These are questions that motivate me to look for big answers that explain the ‘why’s of life, not just the scientific ‘how’. I find truth, peace and purpose in the Christian God.
Where do you find your answers?
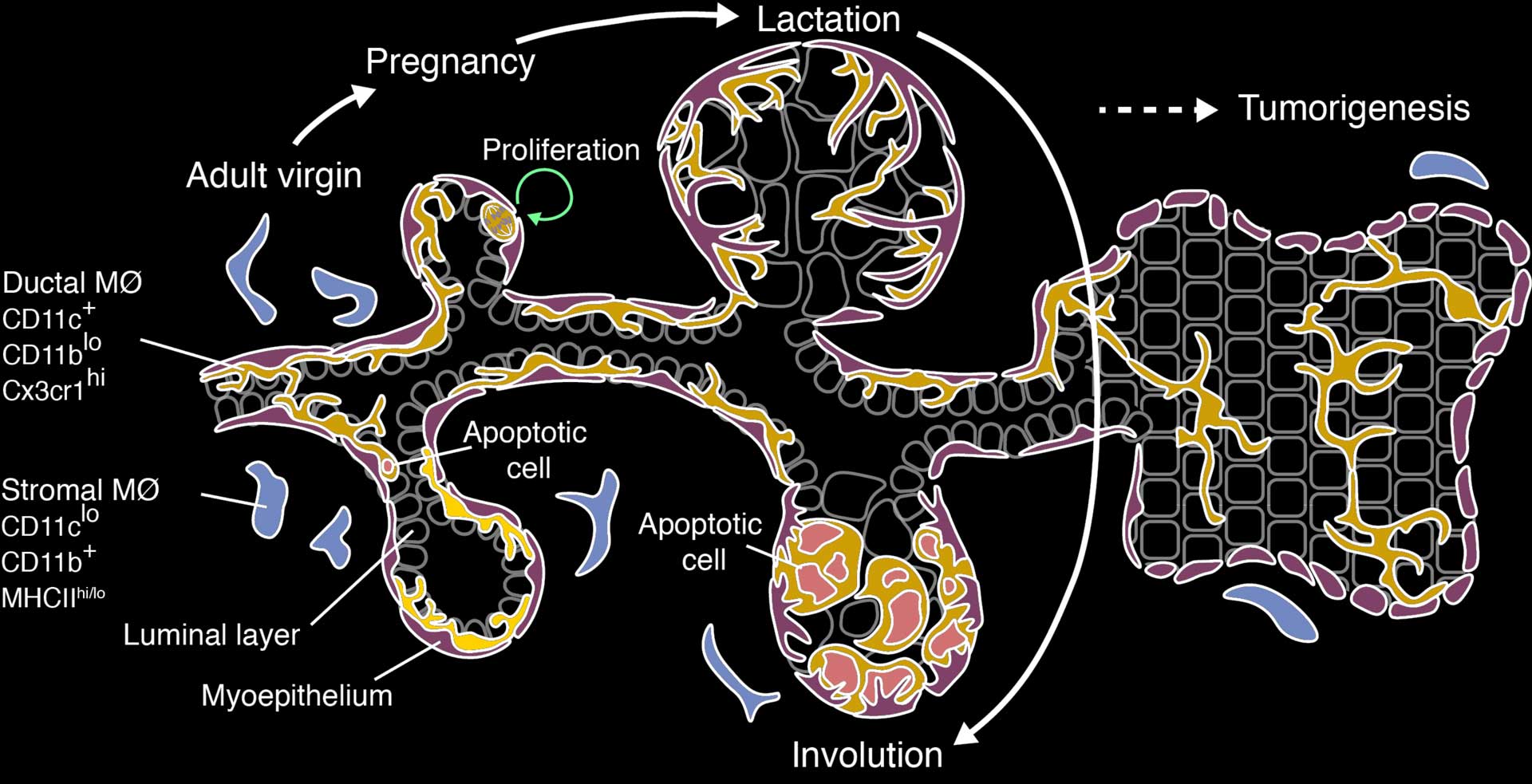
A diagram summarizing the main findings of our study. The symbols on the left are genes that are specifically expressed in the different macrophage (MØ) populations in the breast. DMs proliferate to increase in number in pregnancy, then eat the dying milk-producing cells during involution. DM-like cells continue to form a network within breast cancer.
The research was supported by the National Health and Medical Research Council, the National Breast Cancer Foundation, the Australian Cancer Research Foundation, The Qualtrough Cancer Research Fund, Cure Cancer Australia and the Victorian Government.
Reference: Dawson, C. A., Pal, B., Vaillant. F., Gandolfo, L. C., Liu, Z., Bleriot, C., Ginhoux, F., Smyth, G. K., Lindeman, G. J., Mueller, S. N., Rios, A. C. and Visvader, J. E. (2020), Tissue-resident ductal macrophages survey the mammary epithelium and facilitate tissue remodelling. Nature Cell Biology. https://doi.org/10.1038/s41556-020-0505-0
You may also like
Online presentation – 3D microscopy and new breast immune cells
Navigation
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.